Meet Jeri, a former breast cancer survivor and patient advocate who had the opportunity to avoid a SLNB.
Meet Jeri, a former breast cancer survivor and patient advocate who had the opportunity to avoid a SLNB.
Meet Jeri, a former breast cancer survivor and patient advocate who had the opportunity to avoid a SLNB.
Meet Jeri, a former breast cancer survivor and patient advocate who had the opportunity to avoid a SLNB.
"There was minimal discomfort. It was basically pain-free. I believe that this will be a gamechanger for surgical treatment in breast cancer."
"There was minimal discomfort. It was basically pain-free. I believe that this will be a gamechanger for surgical treatment in breast cancer."
"There was minimal discomfort. It was basically pain-free. I believe that this will be a gamechanger for surgical treatment in breast cancer."
"There was minimal discomfort. It was basically pain-free. I believe that this will be a gamechanger for surgical treatment in breast cancer."
Meet Holly, her concerns about how axillary surgery could affect her lifestyle led her to consider all options available.
Meet Holly, her concerns about how axillary surgery could affect her lifestyle led her to consider all options available.
Meet Holly, her concerns about how axillary surgery could affect her lifestyle led her to consider all options available.
Meet Holly, her concerns about how axillary surgery could affect her lifestyle led her to consider all options available.
"I hope other patients ask about it [Delayed SLNB] because it really is such a simple solution, and has been life-changing for me."
"I hope other patients ask about it [Delayed SLNB] because it really is such a simple solution, and has been life-changing for me."
"I hope other patients ask about it [Delayed SLNB] because it really is such a simple solution, and has been life-changing for me."
"I hope other patients ask about it [Delayed SLNB] because it really is such a simple solution, and has been life-changing for me."
Patient Resources
Patient
Resources
Patient Resources



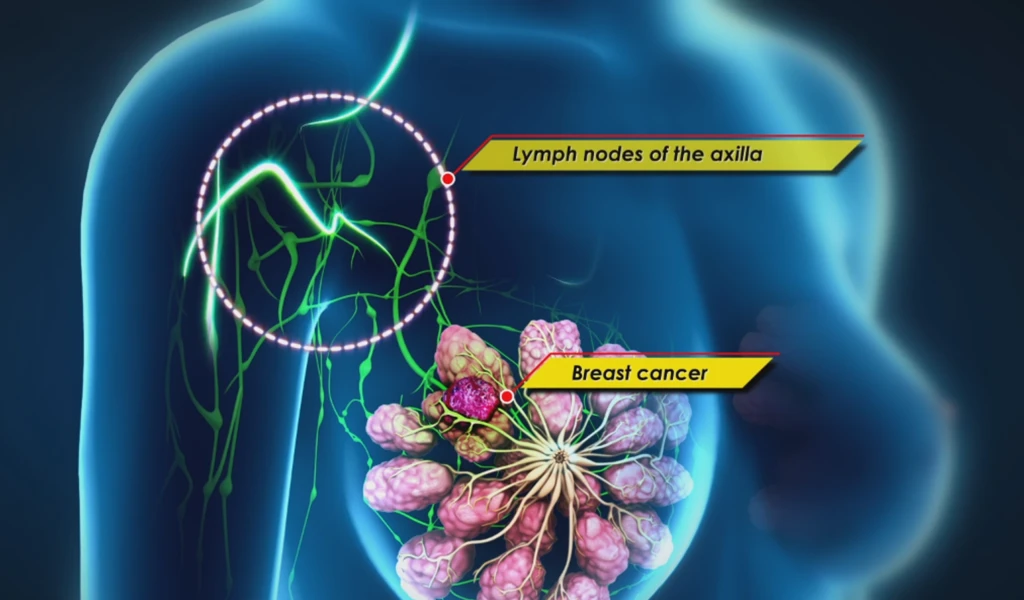
Delayed Sentinel Lymph Node Biopsy
Delayed Sentinel Lymph Node
Biopsy
A delayed sentinel lymph node biopsy is a procedure that allows the surgeon to perform surgery to stage the axilla only when it is truly necessary.



What is the Magtrace® lymphatic tracer?
What is the Magtrace®
lymphatic tracer?
Magtrace® lymphatic tracer is used to mark the first (sentinel) lymph nodes for removal in surgery.
Magtrace® lymphatic tracer is used to mark the first (sentinel) lymph nodes for removal in surgery.
Delayed Sentinel Lymph Node
Biopsy
A delayed sentinel lymph node biopsy is a procedure that allows the surgeon to perform surgery to stage the axilla only when it is truly necessary.
Magtrace® lymphatic tracer is used to mark the first (sentinel) lymph nodes for removal in surgery.
What is the Magtrace®
lymphatic tracer?
Get in touch

'SaveOurNodes' is a patient and physician information campaign on the 'Delayed SLNB' technique, run by Endomag (Endomagnetics Ltd).
© 2024 Endomag (Endomagnetics Ltd). All rights reserved.

'SaveOurNodes' is a patient and physician information campaign on the 'Delayed SLNB' technique, run by Endomag (Endomagnetics Ltd).
© 2024 Endomag (Endomagnetics Ltd). All rights reserved.

'SaveOurNodes' is a patient and physician information campaign on the 'Delayed SLNB' technique, run by Endomag (Endomagnetics Ltd).
© 2024 Endomag (Endomagnetics Ltd). All rights reserved.

'SaveOurNodes' is a patient and physician information campaign on the 'Delayed SLNB' technique, run by Endomag (Endomagnetics Ltd).
© 2024 Endomag (Endomagnetics Ltd). All rights reserved.
© Endomagnetics Ltd (Endomag) is a company registered in England and Wales (No. 06227698).
Registered Office: 330 Cambridge Science Park, Cambridge, CB4 0WN, United Kingdom. VAT Registration No: GB 947 7709 68
© Endomagnetics Ltd (Endomag) is a company registered in England and Wales (No. 06227698).
Registered Office: 330 Cambridge Science Park, Cambridge, CB4 0WN, United Kingdom. VAT Registration No: GB 947 7709 68
© Endomagnetics Ltd (Endomag) is a company registered in England and Wales (No. 06227698).
Registered Office: 330 Cambridge Science Park, Cambridge, CB4 0WN, United Kingdom. VAT Registration No: GB 947 7709 68